Q. How can I get rid of fat underneath my chin?
There are a number of ways that you can reduce the appearance of fat underneath the chin. We always recommend starting with minimally invasive treatments if possible, however there are a number of factors that come into play that may require you to undergo surgical intervention to provide you with your desired results.
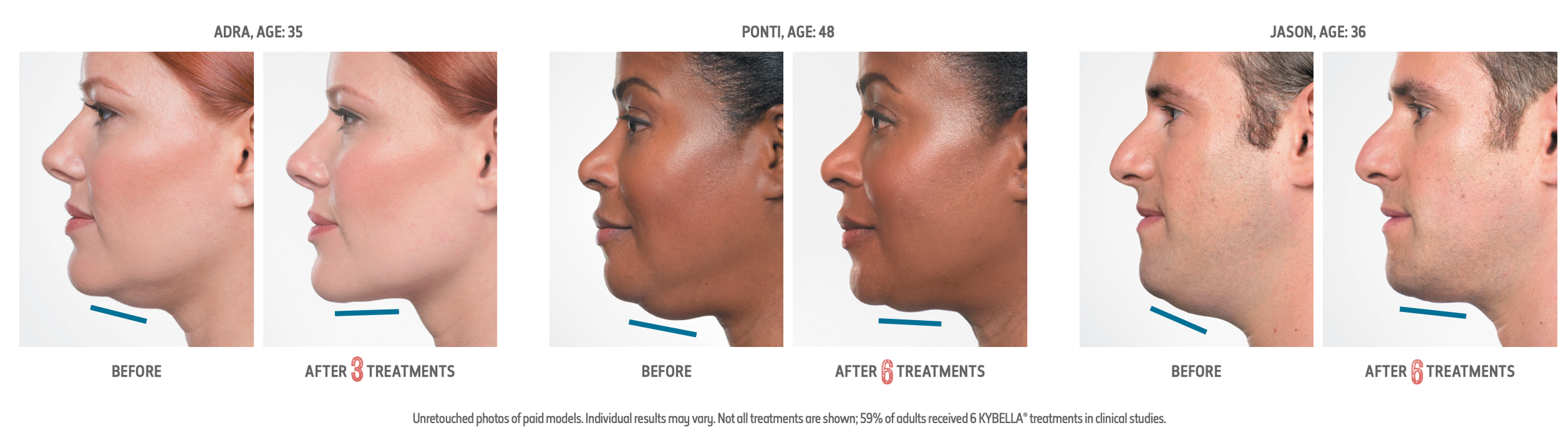
Kybella is an FDA-approved injectable that works to reduce fat under the chin using deoxycholic acid. When injected beneath the chin, Kybella works to break down fat cells, which the body then naturally metabolizes and eliminates over time, resulting in a more contoured and defined appearance.
Depending on your anatomy, your injector may recommend multiple treatments. During each 15-20 minute treatment, you will receive multiple small injections to the target area under your chin, which may result in a slight burning sensation. Your provider may apply numbing cream prior to your treatment to decrease discomfort.
The number of injections will be dependent on the amount of fat you are looking to target to achieve your desired profile. Treatments are administered 4 to 6 weeks apart, this allows time to see the results between each session to gauge the dosage for your upcoming treatments.
Following your treatment you are likely to have swelling, bruising and numbness at the injection site. Read more on what to expect following your treatment by visiting Kybella. As with all cosmetic procedures, it's important to select a board-certified injector to support you on your aesthetic journey. If you are looking for a qualified injector in your area, GET STARTED with our of our patient liaisons for injector recommendations near you.
-
KYBELLA® (deoxycholic acid) injection 10 mg/mL Indication and Important Safety Information
KYBELLA® (deoxycholic acid) injection is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults.
The safe and effective use of KYBELLA® for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended.
IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS
KYBELLA® is contraindicated in the presence of infection at the injection sites.
WARNINGS AND PRECAUTIONS
Marginal Mandibular Nerve Injury
Cases of marginal mandibular nerve injury, manifested as an asymmetric smile or facial muscle weakness, were reported in 4% of subjects in the clinical trials; all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA® should not be injected into or in close proximity to the marginal mandibular branch of the facial nerve.
Dysphagia
Dysphagia occurred in 2% of subjects in the clinical trials in the setting of administration-site reactions, eg, pain, swelling, and induration of the submental area; all cases of dysphagia resolved spontaneously (range 1-81 days, median 3 days). Avoid use of KYBELLA® in patients with current or prior history of dysphagia as treatment may exacerbate the condition.
Injection Site Hematoma/Bruising
In clinical trials, 72% of subjects treated with KYBELLA® experienced hematoma/bruising. KYBELLA® should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the treatment area may occur.
Risk of Injecting Into or in Proximity to Vulnerable Anatomic Structures
To avoid the potential of tissue damage, KYBELLA® should not be injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph nodes, and muscles. Care should be taken to avoid inadvertent injection directly into an artery or a vein as it can result in vascular injury.
Injection Site Alopecia
Cases of injection site alopecia have been reported with administration of KYBELLA®. Onset and duration may vary among individuals and may persist. Consider withholding subsequent treatments until resolution.
Injection Site Ulceration, Necrosis, and Infection
Injections that are too superficial into the dermis may result in skin ulceration and necrosis. Cases of injection site ulceration, necrosis, and infection have been reported with administration of KYBELLA®. Some cases of injection site infection have included cellulitis and abscess requiring antibiotic treatment and incision and drainage. Do not administer KYBELLA® into affected area until complete resolution.
ADVERSE REACTIONS
The most commonly reported adverse reactions in the pivotal clinical trials were: injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and induration.
Please see accompanying KYBELLA® full Prescribing Information or visit https://www.rxabbvie.com/pdf/kybella_pi.pdf
Learn more about additional surgical and non-surgical options for Chin and Neck Contouring.