BOTOX COSMETIC’S NEW FDA APPROVAL: TREATING PLATYSMA BANDS
In a milestone for aesthetic medicine, the FDA has recently approved onabotulinumtoxinA (Botox Cosmetic) to address moderate to severe platysma bands in adults. This new indication, specifically targeting the vertical bands that form along the jaw and neck, provides a non-surgical option for patients who have long been concerned with neck aging but had limited treatment options.
What Are Platysma Bands?
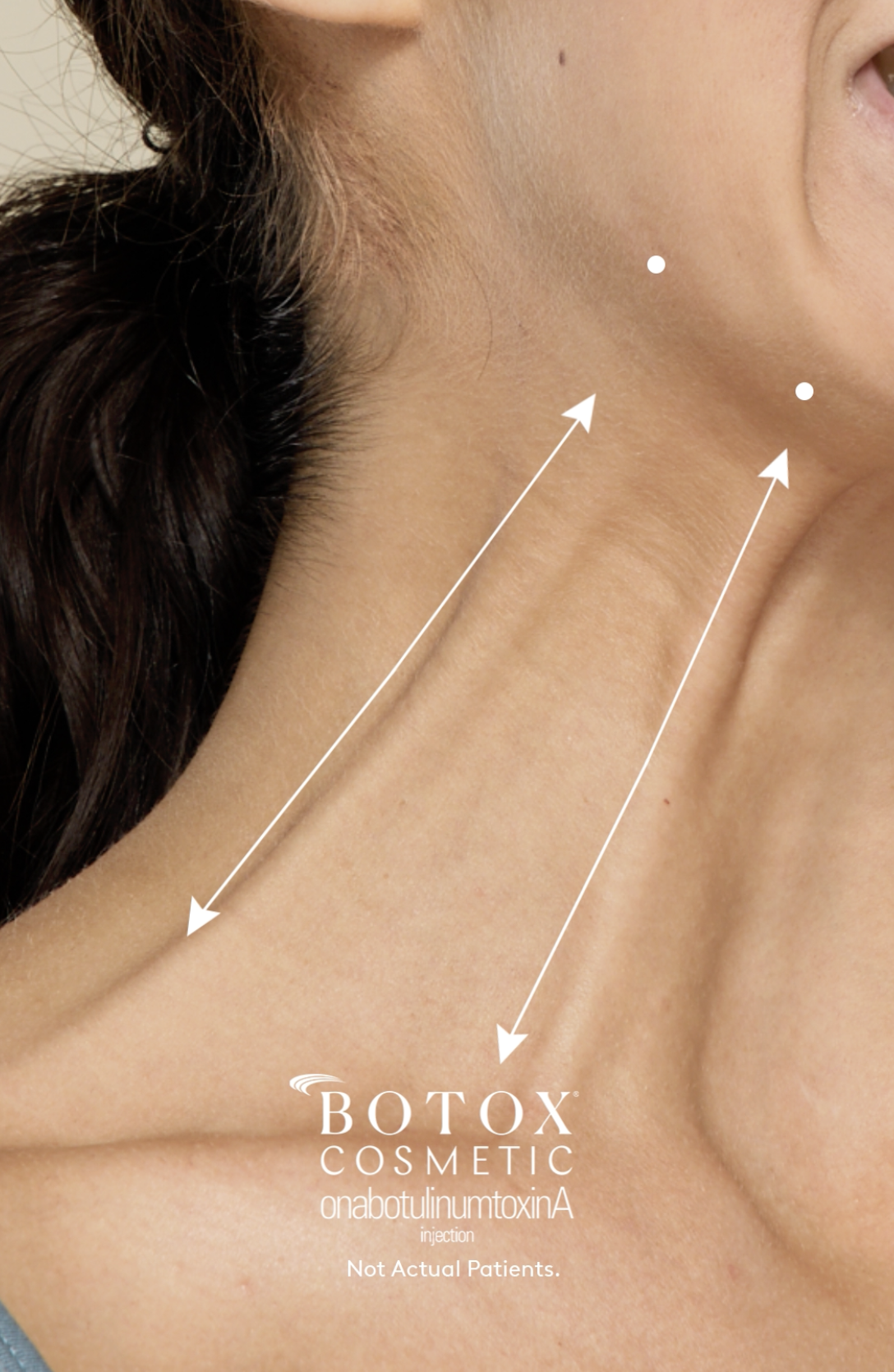
The platysma muscle, a thin layer of muscle extending from the chest to the lower face, can become visible as bands on the neck over time. Aging, repetitive movements, and muscle contractions lead to pronounced platysma bands, giving the neck a less youthful appearance and creating an undefined jawline. The newly FDA-approved Botox Cosmetic treatment directly targets these bands, temporarily reducing muscle activity to soften their appearance.
How Does Botox Cosmetic Treat Platysma Bands?
Botox Cosmetic works by reducing muscle contractions in the treated area. For platysma bands, aesthetic providers inject Botox along the jawline and vertical neck bands using an FDA-approved dose, tailored to the patient's needs—either 26, 31, or 36 units depending on severity. The result is a temporary improvement in the appearance of neck bands, helping to redefine the jawline and soften the overall look of the neck area.
A First in Aesthetic Medicine: Expanding Beyond the Face
With this approval, Botox Cosmetic is now the first and only neurotoxin with FDA clearance to treat four key aesthetic indications: forehead lines, frown lines, crow's feet, and now platysma bands. This new treatment expands the reach of aesthetic injectables beyond the face, setting a precedent in non-invasive rejuvenation for the neck and jawline.
Finding the Right Treatment Plan
The demand for non-surgical options has grown, particularly for neck treatments. Research reveals that many consumers in the U.S. are “extremely or very bothered” by the appearance of platysma bands. Botox Cosmetic’s approval now offers patients a minimally invasive option to address this concern, making it a valuable addition to aesthetic treatment options.
Patients interested in exploring Botox Cosmetic for platysma bands are encouraged to consult with a licensed aesthetic provider to determine the right approach. Treatment should be personalized, taking into account the individual’s anatomy, aesthetic goals, and band severity to achieve optimal results. Click the link to be paired with an ATA Preferred Provider in your area!
-
For Boxed Warning and Medication Guide, see @botoxcosmeticpi.
-
BOTOX® Cosmetic is the first and only FDA-approved treatment to temporarily improve the look of moderate to severe forehead lines, frown lines and forehead lines—and now—BOTOX® Cosmetic GOES BEYOND THE FACE for moderate to severe vertical bands connecting the neck and jaw in adults.
-
BOTOX® Cosmetic (onabotulinumtoxinA) is a prescription medicine that is injected into muscles and used to temporarily improve the look of moderate to severe forehead lines, crow's feet, and frown lines between the eyebrows in adults.
-
Talk to your doctor about BOTOX® Cosmetic and whether it’s right for you. There are risks with this product—the effects of BOTOX® Cosmetic may spread hours to weeks after injection causing serious symptoms. Alert your doctor right away as difficulty swallowing, speaking, breathing, eye problems or muscle weakness can be a sign of a life-threatening condition. If this happens, do not drive a car, operate machinery, or do other dangerous activities. Patients with these conditions before injection are at the highest risk. Swallowing problems may last for several months. Side effects may include allergic reactions, neck and injection-site pain, fatigue and headache. Allergic reactions can include rash, welts, asthma symptoms, and dizziness. Don’t receive BOTOX® Cosmetic if there’s a skin infection. Tell your doctor your medical history, muscle or nerve conditions (including ALS/Lou Gehrig's disease, myasthenia gravis, or Lambert-Eaton syndrome), and medications, including botulinum toxins, as these may increase the risk of serious side effects.
PRO-TIP: A LOOK TO THE FUTURE: LOYALTY & EDUCATION
THROUGH ALLE
As part of this launch, Allergan Aesthetics promotes education and rewards for patients through its Allē loyalty program. With millions of members and a mission to make aesthetic treatments accessible, Allē allows patients to earn points on a range of aesthetic services and treatments, expanding their options for comprehensive aesthetic care. Follow the link below to register for an Allē account and start saving today!
By Lauren Miehl, RN, LE, SLP
ATA Preferred Provider